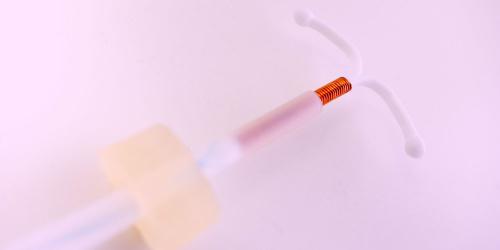
Essure continues to talk about him.
The final sterilization implant, manufactured by the German laboratory Bayer, is no longer marketed throughout the European Union for a period of 3 months, announced in a statement * The National Agency of Products of Health and Medicine (ANSM) Friday, August 4, 2017.
Private CE marking implants
The decision to temporarily suspend the sale was taken by the Irish National Standards Authority of Ireland (NSAI), which did not wish to renew the CE marking of the implant "until all suspense finds an answer, "explained Bayer.
Indeed, to be marketed within the European Union, a medical device must imperatively obtain this marking to materialize its conformity before being put on the market.
Women invited to turn to an alternative
ANSM also asks the manufacturer of the implant to recall all the products currently in stock and specifies that no implant can be placed before November 2, 2017.
In the meantime, the health authority "invites women currently waiting for implantation by this method of permanent sterilization to approach their gynecologist to consider, in consultation, the most appropriate alternative, based on complete information on available contraceptives and possible side effects ".
No questioning of the benefit / risk balance
However, the ANSM wishes to remind the wearers of the implant that this situation does not call into question the benefit / risk balance and that "women with symptoms should consult their doctor so as not to misunderstand an underlying pathology. absence of such a diagnosis, the interest of a withdrawal can be envisaged between the woman concerned and the doctor For women who have no symptoms, which represent the vast majority of women carrying the implant Essure, there is no argument to date to advise the withdrawal. "
Since December 2003, the Essure implant is at the heart of a controversy. Highly criticized by women around the world, some accuse him of causing many serious side effects.
In July 2017, a patient association (RESIST) launched a group action against the manufacturer and a complaint is expected to be filed in September.
* ansm.sante.fr/S-informer/News/The-Marking-CE-of-the-implant-Essure-is-suspendu-for-3-months